INTRODUCING RAPID TESTS TO MEASURE IgG/IgM ANTIBODIES TO THE SARS-COV-2 VIRUS
SSI Diagnostica A/S is exclusively distributor in the Nordics of antibody test from;
- Dynamiker Biotechnology
- CtK Biotech
- AutoBio Diagnostics
The tests are intended for the presumptive qualitative detection of IgM and IgG antibodies to the SARS-CoV-2 virus in patients suspected of a COVID-19 infection.
COVID-19 IgG/IgM Rapid Tests detects anti-SARS-CoV-2 IgG and IgM antibodies in human serum, plasma or whole blood.
The tests can be performed within 15 minutes without the use of time consuming laboratory equipment.
SIMPLE ONE-STEP TEST WITH RAPID RESULTS*
![Illu[2]](https://info.ssidiagnostica.dk/hs-fs/hubfs/Illu%5B2%5D.jpg?width=800&name=Illu%5B2%5D.jpg)
- Rapid results - read after 15 minutes
- Easy to interpret results
- No additional equipment or training needed
- For human plasma, serum or whole blood
* See Instruction For Use for each specific product
PERFORMANCE OF ANTIBODY TESTS
Please see below how the thee Antibody tests performed in a recent evaluation conducted in Denmark.1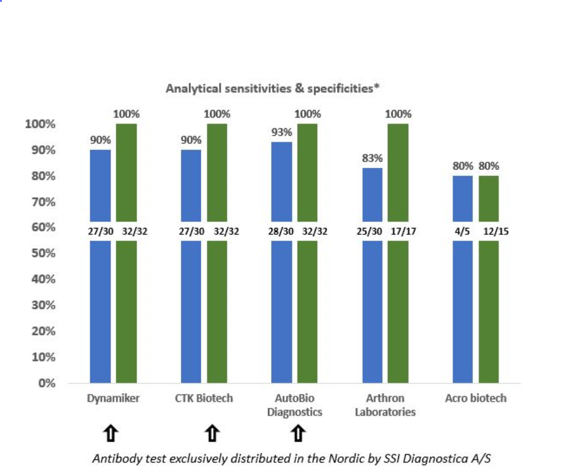
*Data adapted from: Evaluation of nine commercial SARS-CoV-2 immunoassays
https://forskning.ruc.dk/da/publications/evaluation-of-nine-commercial-sars-cov-2-immunoassays
The actual report can be downloaded on the bottom of the page, or you can get in contact with us and hear more.
WHEN TO MEASURE IgG/IgM ANTIBODIES
IgM antibodies are generated initially by the body as a result of infection at about the time symptoms appear.
The rapid test result depends on the time of infection.
IgM immune response is present after 5 days after infection, and will increase over time. Giving that IgM is a marker of early infection.2
Highest IgG immune response is seen after 14 days after infection.2
DETERMINE IMMUNITY WITH RAPID ANTIBODY TESTS
• Determine seroprevalence in a given population
• Define previous exposure
1. RUC (2020): Evaluation of nine commercial SARS-CoV-2 Immunoassays
2. Guo, L. ET. Al; (2020): Clinical Infectious Diseases, https://doi.org/10.1093/cid/ciaa31



